Endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) have been found incompletely removed in various conventional wastewater treatment plants (WWTPs). Wide presence of EDCs and PPCPs in WWTP effluents and in receiving aquatic environments may affect water quality and pose potential risks to aquatic organisms and human health. WWTP effluents have been considered as an important source of micropollutants for aquatic environments; therefore advanced treatment technologies are required to reduce the emission of micropollutants via WWTPs effluents.
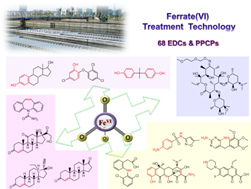
Ferrate(VI) Treatment Technology: We investigated the removal efficiencies of 68 selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) spiked in a wastewater matrix by ferrate (Fe(VI)) and further evaluated the degradation of these micropollutants present in secondary effluents of two wastewater treatment plants (WWTPs) by applying Fe(VI) treatment technology. Fe(VI) treatment resulted in selective oxidation of electron-rich organic moieties of these target compounds, such as phenol, olefin, amine and aniline moieties. But Fe(VI) failed to react with triclocarban, 3 androgens, 7 acidic pharmaceuticals, 2 neutral pharmaceuticals and erythromycin-H2O. Thirty-one target EDCs and PPCPs were detected in the effluents of the two WWTPs with concentrations ranging from 0.2 ± 0.1 ng L-1 to 1156 ± 182 ng L-1. Fe(VI) treatment resulted in further elimination of the detected EDCs and PPCPs during Fe(VI) treatment of the secondary wastewater effluents. The results from this study clearly demonstrated the effectiveness of Fe(VI) treatment as a tertiary treatment technology for a broad spectrum of micropollutants in wastewater.
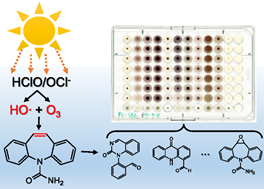
Photolysis of Chlorine Technique: Removal of a persistent antiepileptic drug carbamazepine (CBZ) in aqueous solutions was investigated by using solar photolysis combined with free available chlorine (FAC). The combination of chlorination with simulated or natural sunlight markedly enhanced removal of CBZ in 10 mM phosphate buffer solution (pH 7.0) and river water (pH 7.0) compared with sunlight or FAC alone. Further analysis indicated that the observed enhancements in CBZ removal can be attributed to the in situ hydroxyl radical (HO•) and ozone (O3) production during FAC photolysis. During 70 minutes simulated sunlight photolysis combined with FAC treatment, HO• reaction contributed to 35.8% removal of CBZ and O3 reaction contributed to 40.6% removal, while only 5.3% of CBZ was removed by HOCl reaction. The oxidation products of CBZ, epoxide CBZ, 10,11-dihydro-10,11-dihydroxy CBZ, 1-(2-benzaldehyde)-4-hydro-(1H,3H)-quinazoline-2-one (BQM), 1-(2-benzaldehyde)-(1H,3H)-quinazoline-2,4-dione (BQD) and 4-aldehyde-9-acridone, were mainly formed from the HO• and O3 attack at the double bond on the central heterocyclic ring of CBZ. Formation of these oxidation products did not cause any increase or decrease in toxicity to microbial species tested through Microbial Assay for Toxicity Risk Assessment (MARA). The initial FAC concentration and pH had a major influence on the removal process of CBZ during FAC photolysis, while temperature had a minor effect only. The combination of chlorination with natural sunlight could provide an effective approach for removal of CBZ and other contaminants during water treatment.
References:
(1) Yang, B., Kookana, R.S., Williams, M., Du, J., Doan, H. and Kumar, A. (2016) Removal of carbamazepine in aqueous solutions through solar photolysis of free available chlorine. Water Research 100, 413-420.
(2) Yang, B., Ying, G.-G., Chen, Z.-F., Zhao, J.-L., Peng, F.-Q. and Chen, X.-W. (2014) Ferrate(VI) oxidation of tetrabromobisphenol A in comparison with bisphenol A. Water Research 62, 211-219.
(3) Yang, B. and Ying, G.-G. (2013) Oxidation of benzophenone-3 during water treatment with ferrate(VI). Water Research 47(7), 2458-2466.
(4) Yang, B., Ying, G.-G., Zhao, J.-L., Liu, S., Zhou, L.-J. and Chen, F. (2012) Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate(VI) treatment of secondary wastewater effluents. Water Research 46(7), 2194-2204.
(5) Yang, B., Ying, G.-G., Zhang, L.-J., Zhou, L.-J., Liu, S. and Fang, Y.-X. (2011) Kinetics modeling and reaction mechanism of ferrate(VI) oxidation of benzotriazoles. Water Research 45(6), 2261-2269.